Covid-19 Vaccine Resources - COVID-19 Resources | MNeuronet | Michigan Medicine ... - A covid‑19 vaccine is a vaccine intended to provide acquired immunity against severe acute respiratory syndrome coronavirus 2 (sars‑cov‑2), the virus causing coronavirus disease 2019.
Covid-19 Vaccine Resources - COVID-19 Resources | MNeuronet | Michigan Medicine ... - A covid‑19 vaccine is a vaccine intended to provide acquired immunity against severe acute respiratory syndrome coronavirus 2 (sars‑cov‑2), the virus causing coronavirus disease 2019.. This is an exciting time. Currently, we are in phase 1a of pennsylvania's vaccine rollout. The vaccines met fda's rigorous scientific standards for safety, effectiveness, and manufacturing quality needed to support emergency use authorization (eua). By henrik pettersson, byron manley, sergio hernandez and deidre mcphillips, cnn. Vaccines are safe and effective and the best way to protect you and those around you from serious illnesses.
An overview for providers (pdf, march 26): Pregnant women who are inadvertently immunised should be notified to the vaccine in pregnancy surveillance programme. Vaccine faqs for health care providers (pdf, february 19): The vaccines met fda's rigorous scientific standards for safety, effectiveness, and manufacturing quality needed to support emergency use authorization (eua). This is an exciting time.
This link will open in a new window.
This call centre does not book appointments for pharmacies. The vaccines met fda's rigorous scientific standards for safety, effectiveness, and manufacturing quality needed to support emergency use authorization (eua). By henrik pettersson, byron manley, sergio hernandez and deidre mcphillips, cnn. As part of our targeted vaccine efforts, we also are vaccinating teachers and child care workers and. Visit public health unit for more information. Currently, we are in phase 1a of pennsylvania's vaccine rollout. Several pharmaceutical companies have published their vaccine trial protocols. This unusually transparent action during a major drug trial deserves praise, close inspection of the protocols raises surprising concerns. A covid‑19 vaccine is a vaccine intended to provide acquired immunity against severe acute respiratory syndrome coronavirus 2 (sars‑cov‑2), the virus causing coronavirus disease 2019. This is an exciting time. An overview for providers (pdf, march 26): As of july 2020, more than 150 vaccines are being developed in different laboratories, but none of them have completed clinical trials yet. Vaccines are safe and effective and the best way to protect you and those around you from serious illnesses.
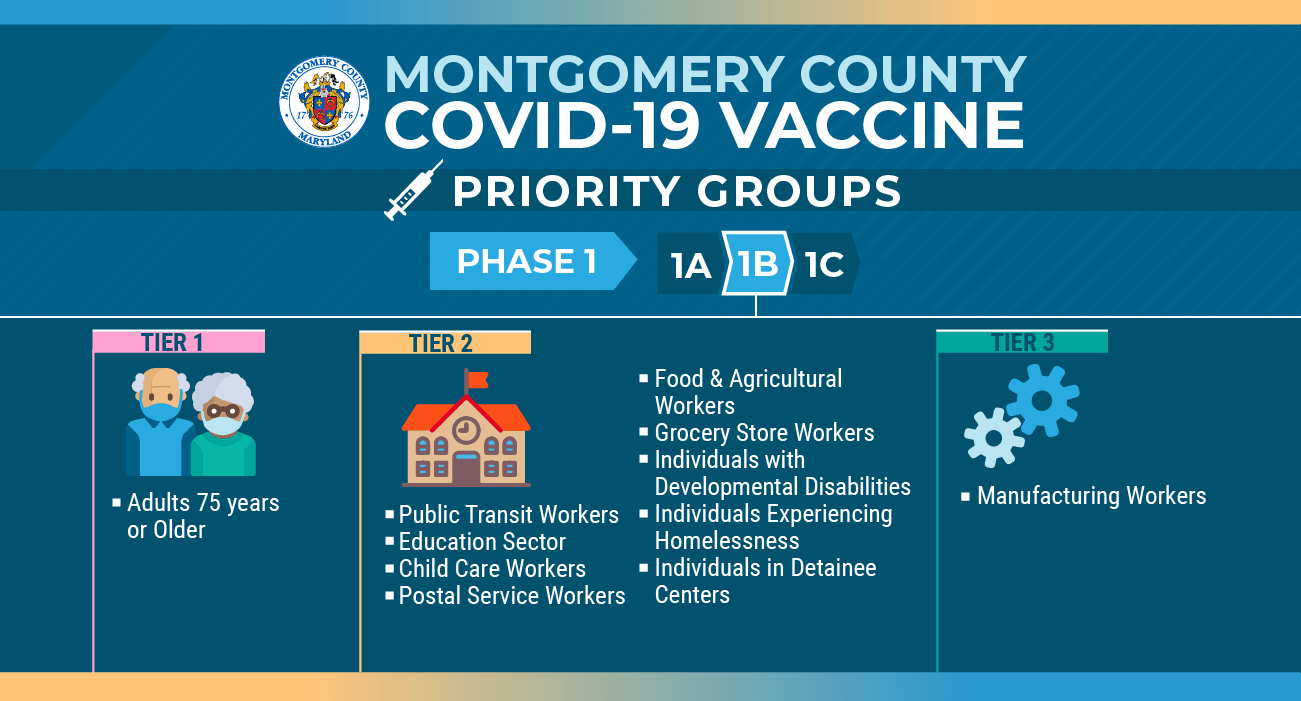
While the vaccine supply is limited, it is necessary to prioritize who gets the vaccine, with people who are at highest risk of fact v. Visit public health unit for more information. This link will open in a new window. A covid‑19 vaccine is a vaccine intended to provide acquired immunity against severe acute respiratory syndrome coronavirus 2 (sars‑cov‑2), the virus causing coronavirus disease 2019. Pregnant women who are inadvertently immunised should be notified to the vaccine in pregnancy surveillance programme.

As of july 2020, more than 150 vaccines are being developed in different laboratories, but none of them have completed clinical trials yet.
Pregnant women who are inadvertently immunised should be notified to the vaccine in pregnancy surveillance programme. As part of our targeted vaccine efforts, we also are vaccinating teachers and child care workers and. This call centre does not book appointments for pharmacies. A covid‑19 vaccine is a vaccine intended to provide acquired immunity against severe acute respiratory syndrome coronavirus 2 (sars‑cov‑2), the virus causing coronavirus disease 2019. All content article title authors keywords abstract article title, abstract, keywords. It was first reported in december 2019. The vaccines met fda's rigorous scientific standards for safety, effectiveness, and manufacturing quality needed to support emergency use authorization (eua). Vaccines are safe and effective and the best way to protect you and those around you from serious illnesses. Find resources to guide vaccination planning and communication resources for specific populations. Vaccine faqs for health care providers (pdf, february 19): By henrik pettersson, byron manley, sergio hernandez and deidre mcphillips, cnn. Johns hopkins' maryland hospitals continue to vaccinate our eligible patients and work with partners in the community to distribute the vaccine to eligible individuals based on state guidance. Presentation slides with information on vaccine development, clinical considerations, safety monitoring, distribution and counseling patients.
While the vaccine supply is limited, it is necessary to prioritize who gets the vaccine, with people who are at highest risk of fact v. Pregnant women who are inadvertently immunised should be notified to the vaccine in pregnancy surveillance programme. This resource site contains information and resources for covid‑19 vaccine providers and local health entities related to covid‑19 vaccine providers will also have access to a data dashboard with information about vaccine allocation, supply chain, and administration for their provider organization. Johns hopkins' maryland hospitals continue to vaccinate our eligible patients and work with partners in the community to distribute the vaccine to eligible individuals based on state guidance. This call centre does not book appointments for pharmacies.

Vaccine faqs for health care providers (pdf, february 19):
Visit public health unit for more information. This unusually transparent action during a major drug trial deserves praise, close inspection of the protocols raises surprising concerns. The vaccines met fda's rigorous scientific standards for safety, effectiveness, and manufacturing quality needed to support emergency use authorization (eua). Vaccine faqs for health care providers (pdf, february 19): This resource site contains information and resources for covid‑19 vaccine providers and local health entities related to covid‑19 vaccine providers will also have access to a data dashboard with information about vaccine allocation, supply chain, and administration for their provider organization. As of july 2020, more than 150 vaccines are being developed in different laboratories, but none of them have completed clinical trials yet. Find resources to guide vaccination planning and communication resources for specific populations. A covid‑19 vaccine is a vaccine intended to provide acquired immunity against severe acute respiratory syndrome coronavirus 2 (sars‑cov‑2), the virus causing coronavirus disease 2019. By henrik pettersson, byron manley, sergio hernandez and deidre mcphillips, cnn. It was first reported in december 2019. This call centre does not book appointments for pharmacies. An overview for providers (pdf, march 26): All content article title authors keywords abstract article title, abstract, keywords.
Komentar
Posting Komentar